Molar Ratio Calculator
Our molar ratio calculator will help you determine the molar ratio between the different chemicals reacting and the different chemicals produced during the reaction. It can also help you determine the mass or the number of moles of each chemical required to perform the reaction. Equipped with that knowledge, you can find the limiting agent in the reaction or which chemical is available in excess.
Are you wondering what is a molar ratio and how to calculate it? Do you what to learn the importance of a molar ratio? Join us in this article as we discuss the definition of a molar ratio, how to determine it using the molar ratio formula, and how to use it to get more information from your balanced chemical equation.
The molar ratio is different from a mole fraction. Our mole fraction calculator will serve you if you're interested in the mole fraction of your solution.
What is a molar ratio? What is molar ratio formula?
A molar ratio is between the number of moles (or molecules) of reactants consumed and the number of moles (or molecules) of products generated in a chemical reaction. You can also express it as the ratio of the number of moles (or molecules) of one reactant required to completely react with another reactant or one product produced to another product.
For example, during ammonia production, if 30 moles of hydrogen react entirely with 10 moles of nitrogen to give 20 moles of ammonia, then you can write the molar ratio between different participants in the reaction as:
If you want to find how much of each element is present in a compound, our percent composition calculator can help you.
How to calculate molar ratio?
To determine the molar ratio between any two elements or compounds in a chemical reaction:
- Balance the chemical reaction.
- Obtain the coefficients of the corresponding elements or compounds in the balanced equation.
- Calculate the ratio between these coefficients.
In other words, the formula for molar ratio between any two elements or compounds is as simple as:
where:
- – Any element or compound reacting or produced in the reaction;
- – Any other element or compound reacting or produced in the reaction;
- – Coefficient of in the balanced chemical reaction; and
- – Coefficient of in the balanced chemical reaction.
The coefficient in a balanced reaction is useful to determine the molar ratio because the coefficient represents the number of molecules of that element (or compound) needed for the reaction to happen without leaving behind any excess reactants.
For example, consider the following balanced chemical equation:
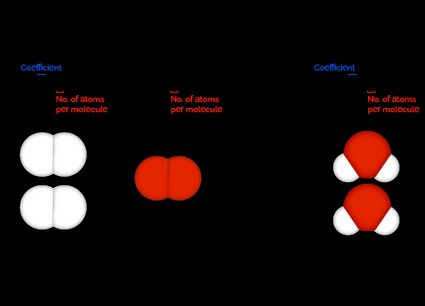
From the coefficients in this balanced chemical reaction, we can derive the following information:
- For every two molecules of , it takes one molecule of to react completely;
- For every two molecules of reacting, two molecules of are formed; and
- For every molecule of reacting, two molecules of are formed.
This result also means that if we had twenty molecules of hydrogen, we would need ten molecules of oxygen to completely react with it to produce twenty molecules of water.
In every balanced reaction, this proportion between the number of molecules stands between different reactants and products. One mole of a substance is simply the aggregation of number of atoms or molecules. Use our avogadro's number calculator to understand this further.
Significance of a molar ratio and its equation
Now that you understand how to determine a chemical reaction's molar ratio, let's go on to learn its importance.
Once you have the molar ratio, you can figure out how many moles of each item you need for the reaction to take place completely. For example, if the molar ratio between two reactants is 2:3, and you have of the first reactant, then the number of moles of the second reactant required is given by:
You can use the molar ratio and molecular weight of the molecules to determine the mass of the elements (or compounds) required to complete the reaction. Use our grams to moles calculator calculator and mole calculator to understand how molecular weight and molar mass of a compound are related and how to convert the number of moles to the required compound's mass. Hence, once you know how to find the molar ratio and number the moles required, you can "convert" the molar ratio to grams or "mass" ratio:
Since the molar ratio helps determine how much of each substance is required to complete the reaction, you can also use it to find out which substance is the limiting reagent and which substance is in excess. For example, we've seen that the molar ratio equation between hydrogen and oxygen in water production is 2:1. If we had 12 mol of hydrogen for 10 mol of oxygen, we immediately know hydrogen is the limiting reagent because the reaction demands that we have 20 mol of hydrogen. We can also phrase that oxygen is in excess by 4 mol since only 6 mol can react with 12 mol of hydrogen.
How do you find molar ratio using this online molar ratio calculator?
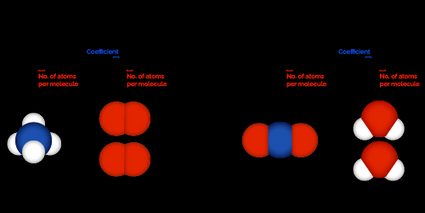
This online molar ratio calculator can handle up to five reactants and five products. Additionally, there are three types of calculations to choose from, explained below. Keep in mind that in all these cases, the resultant molar ratio is displayed as a table at the very bottom of the calculator.
-
Calculating the molar ratio from a balanced reaction is the most straightforward method. In this molar ratio calculator, first set Select calculation type to Calculate molar ratio. Then carefully enter the coefficients of each reactant and product in the corresponding Coefficient field. You will get the resultant molar ratio at the very bottom as a table.
-
Once you have the molar ratio, you can determine the number of moles of each reactant and product required to complete the reaction. Conversely, if you know the number of moles needed, you can compute the molar ratio. In this calculator, first set Select calculation type to Calculate molar ratio and moles and carefully enter the coefficients or the number of moles in the corresponding fields. You will get the resultant molar ratio at the very bottom as a table.
-
It is possible to calculate the mass of each reactant and product required if you know the molar ratio, number of moles required, and molecular weight of each reactant and product. In this calculator, first set Select calculation type to Calculate molar ratio, moles, and mass. Then enter the coefficients, the number of moles, and the mass in the corresponding fields.
-
To evaluate the molecular weight, we need to know each element in the compound along with the number of atoms per molecule. For example, for , enter in the first Atoms per molecule field and select as its unit, and enter in the second Atoms per molecule field and select as its unit. This calculator can support up to five elements per reactant or product.
-
We recommend you utilize the Atoms per molecule field to calculate the molecular weight, but you're free to manually enter any value in the molecular weight field. The calculator may continue to prompt you with a message "Number of atoms per molecule has to be a positive integer." This is because this calculator uses atomic masses as listed in the , and the values you're using may be slightly different.
FAQ
How to calculate molar ratio from molecular weight?
To determine the molar ratio from the molecular weight, you need the mass of each substance.
- Convert the molecular weight of the first substance into its molar mass.
- Divide the mass of the first substance by its molar mass to obtain the number of moles used (or produced) in the reaction.
- Repeat steps one and two to the second substance to obtain the number of moles used (or produced) in the reaction.
- Calculate the ratio between the number of moles of the two substances to obtain their molar ratio.
What is the molar ratio between sodium and chlorine in the formation of table salt?
2:1. For every two moles of sodium, one mole of chlorine is required to form two moles of sodium chloride, commonly known as table salt.
How do I convert molar ratio to volume ratio?
The molar ratio and volume ratio are equal if all the gases reacting and produced are at the same temperature and pressure.
- Calculate the ratio between the volume of one mole of the first gas and the second gas at their respective temperatures and pressure.
- Multiply this ratio with the molar ratio to obtain the volume ratio.
- Be proud of yourself for having solved a complex problem.
How to calculate molar ratio from grams?
To calculate the molar ratio from the mass of the reactants (or products), follow these simple steps:
- Calculate the molar mass of each substance by evaluating their molecular weight.
- Divide the mass of the first substance by its molar mass to obtain the number of moles used (or produced) in the reaction.
- Repeat step one for the second substance to obtain the number of moles used (or produced) in the reaction.
- Calculate the ratio between the number of moles of the two substances to obtain their molar ratio.